
時(shí)間:2021-03-04
作者:易科泰
點(diǎn)擊量:
簡(jiǎn)介:
FP-leaf葉夾式植物光譜與葉綠素?zé)晒鉁y(cè)量包用于測(cè)量葉片水平的植物葉綠素?zé)晒?、葉片反射光譜及光譜指數(shù)等,,包括手持式葉綠素?zé)晒鉁y(cè)量?jī)x和植物反射光譜測(cè)量?jī)x,。適于野外大量樣品的快速檢測(cè),廣泛應(yīng)用于植物脅迫響應(yīng)、除草劑檢測(cè),,生態(tài)毒理生物檢測(cè),、植物反射光譜測(cè)量、色素組成變化,、氮素含量變化,、產(chǎn)量估測(cè)、生態(tài)學(xué),、分子生物學(xué)等,。

測(cè)得的數(shù)據(jù)以圖形或數(shù)據(jù)表的形式實(shí)時(shí)顯示在儀器的顯示屏上。這些數(shù)據(jù)都可以?xún)?chǔ)存在儀器的內(nèi)存里并傳輸?shù)诫娔X里,。測(cè)量?jī)x由可充電鋰電池供電,,不需要使用電腦即可獨(dú)立進(jìn)行測(cè)量。測(cè)量?jī)x配備全彩色觸屏顯示器,、內(nèi)置光源,、內(nèi)置GPS和用于固定樣品的無(wú)損葉夾,。
應(yīng)用領(lǐng)域
適用于光合作用研究和教學(xué),植物及分子生物學(xué)研究,,農(nóng)業(yè),、林業(yè),生物技術(shù)領(lǐng)域等,。研究?jī)?nèi)容涉及光合活性,、脅迫響應(yīng)、農(nóng)藥藥效測(cè)試,、突變篩選,、色素含量評(píng)估等。

功能特點(diǎn)
技術(shù)參數(shù)
1. 測(cè)量參數(shù)及程序
1.1 葉綠素?zé)晒鉁y(cè)量包括F0、Ft,、Fm,、Fm’、QY,、QY_Ln,、QY_Dn、NPQ,、Qp,、Rfd、PAR(限PAR型號(hào)),、Area,、Mo、Sm,、PI,、ABS/RC等50多個(gè)葉綠素?zé)晒鈪?shù)
1.2 葉綠素?zé)晒釵JIP–test包括F0、Fj,、Fi,、Fm、Fv,、Vj,、Vi、Fm/F0,、Fv/F0,、Fv/Fm、Mo,、Area,、Fix Area、Sm,、Ss,、N、Phi_Po,、Psi_o,、Phi_Eo、Phi–Do,、Phi_Pav,、PI_Abs、ABS/RC,、TRo/RC,、ETo/RC、DIo/RC等
1.3 葉綠素?zé)晒鉁y(cè)量程序:Ft,、QY,、OJIP、NPQ1,、NPQ2,、NPQ3、LC1、LC2,、LC3,、PAR(限PAR型號(hào))、Multi無(wú)人值守自動(dòng)監(jiān)測(cè)
1.4 植被反射指數(shù):NDVI,、SR,、綠度指數(shù)、MCARI,、TCARI,、TVI、ZMI,、SRPI,、NPQI、PRI,、NPCI,、Carter指數(shù)、SIPI,、GM1,、SR、MCARI1,、OSAVI,、MCARI、Ctr2,、GM2(視具體型號(hào)而定)
2. 手持式葉綠素?zé)晒鉁y(cè)量單元:
2.1 葉夾類(lèi)型:固定葉夾式,、分離葉夾式、探頭式等
2.2 PAR傳感器:80o入射角余弦校正,,讀數(shù)單位μmol(photons)/m2.s,,可顯示讀數(shù),檢測(cè)范圍400-700 nm
2.3  測(cè)量光:每測(cè)量脈沖最高0.09μmol(photons)/m2.s,,10-100%可調(diào)
測(cè)量光:每測(cè)量脈沖最高0.09μmol(photons)/m2.s,,10-100%可調(diào)
2.4 光化學(xué)光:10-1000μmol(photons)/m2.s可調(diào)
2.5 飽和光:最高3000μmol(photons)/m2.s,,11-100%可調(diào)
2.6 光源:標(biāo)準(zhǔn)配置藍(lán)光455nm,可根據(jù)需求配備不同波長(zhǎng)的LED光源
2.7 尺寸大?。撼銛y,,手機(jī)大小,134×65×33mm(不包括探頭),,重量?jī)H188g
2.8 數(shù)據(jù)存儲(chǔ):容量16Mb,,可存儲(chǔ)149000數(shù)據(jù)點(diǎn)
2.9 顯示與操作:圖形化顯示,雙鍵操作,,待機(jī)5分鐘自動(dòng)關(guān)閉
2.10 供電:2000mA可充電鋰電池,,USB充電,,可連續(xù)工作48小時(shí),低電報(bào)警
2.11 工作條件:0–55℃,,0–95%相對(duì)濕度(無(wú)凝結(jié)水)
2.12 存貯條件:-10–60℃,,0–95%相對(duì)濕度(無(wú)凝結(jié)水)
2.13 通訊方式:藍(lán)牙 + USB雙通訊模式,藍(lán)牙在20m距離最大傳輸速度3Mbps
2.14 GPS模塊:內(nèi)置,,最高精度1.5m
2.15 軟件:FluorPen1.1專(zhuān)用軟件,,用于數(shù)據(jù)下載、分析和圖表顯示,,輸出Excel數(shù)據(jù)文件及熒光動(dòng)力學(xué)曲線(xiàn)圖
3. 手持式植物反射光譜單元
3.1 光譜檢測(cè)范圍:
PolyPen RP 410 UVIS光譜響應(yīng)范圍為380-790nm
PolyPen RP 410 NIR光譜響應(yīng)范圍為640-1050nm

3.2 光源:氙氣白熾燈380-1050nm
3.3 光譜響應(yīng)半寬度:8nm
3.4 光譜雜散光:-30dB
3.5 光學(xué)孔徑:7mm
3.6 掃描速度:約100ms
3.7 觸控屏:240×320像素,65535色
3.8 內(nèi)存:16MB(可存儲(chǔ)4000組以上測(cè)量數(shù)據(jù))
3.9 系統(tǒng)數(shù)據(jù):16位數(shù)模轉(zhuǎn)換
3.10 動(dòng)態(tài)范圍:高增益 1:4300,;低增益 1:13000
3.11 內(nèi)置GPS模塊:最大精度<1.5m
3.12 通訊方式:USB
3.13 軟件功能:自動(dòng)計(jì)算內(nèi)置植被指數(shù),、計(jì)算用戶(hù)自定義植被指數(shù)、實(shí)時(shí)顯示數(shù)據(jù)圖和數(shù)據(jù)表,、數(shù)據(jù)導(dǎo)出為Excel,、GPS地圖、固件升級(jí),,Windows XP及以上系統(tǒng)適用
3.14 光譜反射標(biāo)準(zhǔn)配件(選配):提供最高的漫反射值(99%),。光譜平面涵蓋UV-VIS-NIR光譜,保證+/-1%的光學(xué)平面,。用于光源和檢測(cè)器的校準(zhǔn),。
3.15 尺寸:15×7.5×4cm
3.16 重量:300g
3.17 外殼:防水濺外殼
3.18 電池:2600mAh可充電鋰電池,通過(guò)USB接口連接電腦充電
3.19 續(xù)航時(shí)間:可連續(xù)測(cè)量48小時(shí)
3.20 工作條件:溫度0~55℃,,相對(duì)濕度0-95%(無(wú)冷凝水)
3.21 存放條件:溫度-10~60℃,,相對(duì)濕度0-95%(無(wú)冷凝水)
應(yīng)用案例 1:
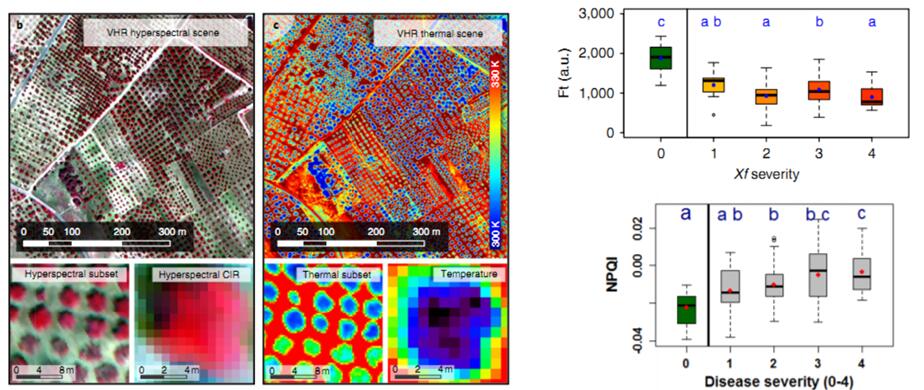
歐盟委員會(huì)聯(lián)合研究中心通過(guò)無(wú)人機(jī)遙測(cè)技術(shù)研究葉緣焦枯病菌在橄欖樹(shù)中的感染。同時(shí)通過(guò)FluorPen葉綠素?zé)晒鈨x和RP400光譜儀直接檢測(cè)葉片的葉綠素?zé)晒夂头瓷涔庾V植被指數(shù),,用于對(duì)照修正無(wú)人機(jī)遙測(cè)數(shù)據(jù),。研究結(jié)果發(fā)表在《Nature Plants》(Zarco-Tejada,2018),。
應(yīng)用案例 2:
水稻灌漿期的夜間高溫會(huì)顯著影響水稻的產(chǎn)量,。捷克科學(xué)院全球變化研究中心與國(guó)際水稻研究所合作研究夜間高溫對(duì)成熟水稻穗光學(xué)特性的變化追蹤。研究者使用FluorPen手持式葉綠素?zé)晒鈨x測(cè)量了光合系統(tǒng)有效光化學(xué)效率ΦII(也稱(chēng)為有效量子產(chǎn)額QY或ΦPSII)和穩(wěn)態(tài)熒光Fs,。同時(shí)使用PolyPen手持式植物反射光譜測(cè)量?jī)x的前期型號(hào)WinePen測(cè)量了反射光譜曲線(xiàn),,并計(jì)算了PRI、mSR705,、mND705,、R470/R570、R520/R675等9項(xiàng)植被指數(shù),。這些植被指數(shù)與水稻葉片/穗的光合能力,、穩(wěn)態(tài)熒光、葉綠素濃度等緊密相關(guān)(Gil-Ortiz R et al. 2020)。
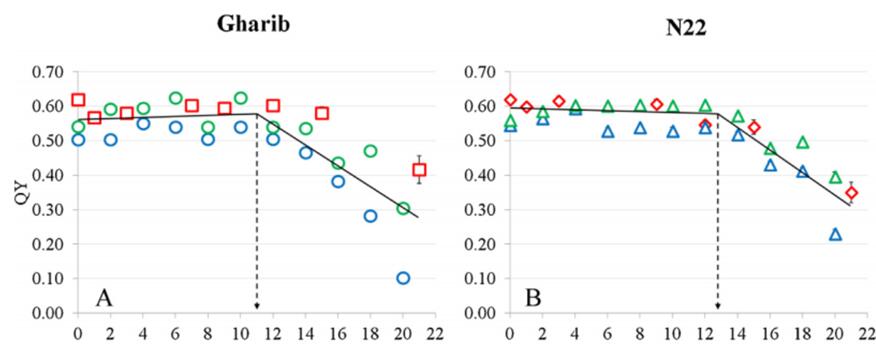
圖1. 不同品種水稻的有效量子產(chǎn)額QY時(shí)間趨勢(shì)
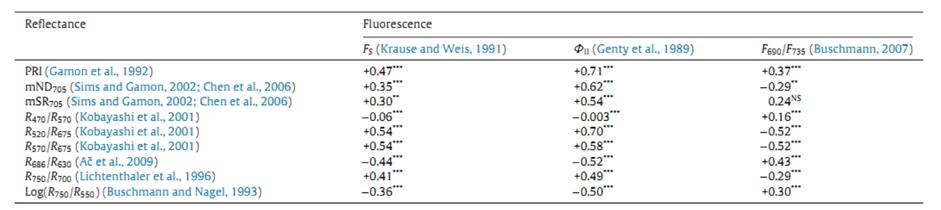
圖2. 反射植被指數(shù)與葉綠素?zé)晒鈪?shù)的線(xiàn)性回歸系數(shù)
參考文獻(xiàn)
1. Singh, S., Mohan Prasad, S. & Pratap Singh, V. Additional calcium and sulfur manages hexavalent chromium toxicity in Solanum lycopersicum L. and Solanum melongena L. seedlings by involving nitric oxide. Journal of Hazardous Materials 398, 122607 (2020).
2. Ariyarathna, R. a. I. S., Weerasena, S. L. & Beneragama, C. K. Application of Polyphasic OJIP Chlorophyll Fluorescent Transient Analysis as an Indicator for Testing of Seedling Vigour of Common Bean (Phaseolus vulgaris L.). Tropical Agricultural Research 31, 106–115 (2020).
3. Prity, S. A. et al. Arbuscular mycorrhizal fungi mitigate Fe deficiency symptoms in sorghum through phytosiderophore-mediated Fe mobilization and restoration of redox status. Protoplasma (2020) doi:10.1007/s00709-020-01517-w.
4. Rahman, M. A. et al. Arbuscular Mycorrhizal Symbiosis Mitigates Iron (Fe)-Deficiency Retardation in Alfalfa (Medicago sativa L.) Through the Enhancement of Fe Accumulation and Sulfur-Assisted Antioxidant Defense. International Journal of Molecular Sciences 21, 2219 (2020).
5. Vitorino, L. C. et al. Biocontrol Potential of Sclerotinia sclerotiorum and Physiological Changes in Soybean in Response to Butia archeri Palm Rhizobacteria. Plants 9, 64 (2020).
6. Kasampalis, D. S., Tsouvaltzis, P. & Siomos, A. S. Chlorophyll fluorescence, non-photochemical quenching and light harvesting complex as alternatives to color measurement, in classifying tomato fruit according to their maturity stage at harvest and in monitoring postharvest ripening during storage. Postharvest Biology and Technology 161, 111036 (2020).
7. Soares, J. S., Santiago, E. F. & Sorgato, J. C. Conservation of Schomburgkia crispa Lindl. (Orchidaceae) by reintroduction into a fragment of the Brazilian Cerrado. Journal for Nature Conservation 53, 125754 (2020).
8. Poblete, T. et al. Detection of Xylella fastidiosa infection symptoms with airborne multispectral and thermal imagery: Assessing bandset reduction performance from hyperspectral analysis. ISPRS Journal of Photogrammetry and Remote Sensing 162, 27–40 (2020).
9. Chiluwal, A. et al. Deterioration of ovary plays a key role in heat stress-induced spikelet sterility in sorghum. Plant, Cell & Environment 43, 448–462 (2020).
10.Maai, E., Nishimura, K., Takisawa, R. & Nakazaki, T. Diurnal changes in chloroplast positioning and photosynthetic traits of C4 grass finger millet. Plant Production Science 0, 1–13 (2020).
11.De Micco, V. et al. Dust accumulation due to anthropogenic impact induces anatomical and photochemical changes in leaves of Centranthus ruber growing on the slope of the Vesuvius volcano. Plant Biol J 22, 93–102 (2020).
12.Gil-Ortiz R et al. 2020. New Eco-Friendly Polymeric-Coated Urea Fertilizers Enhanced Crop Yield in Wheat. Agronomy 10: 438
13.Zarco-Tejada, P. J., Camino, C., Beck, P. S. A., Calderon, R., Hornero, A., et al. 2018. Previsual symptoms of Xylella fastidiosa infection revealed in spectral plant-trait alterations. Nature Plants, 4(7), 4 ts, 4(7), 432–439.
14.Poblete, T., Camino, C., Beck, P. S. A.,A., Hornero, A., et al. 2020. Detection of Xylella fastidiosa in fastidiosa infection symptoms with airborne multispectr tral and thermal imagery: Assessing bandset redu eduction performance from hyperspectral analysis. ISPRS Journal of urnal of Photogrammetry and Remote Sensing, 162, 27–40.
15.Junker L. V., Rascher U., Jaenicke H., et al. 2019. Detection of plant stress responses in aphid-infested lettuce using non-invasive detection methods. Integrated Protection in Field Vegetables IOBC OBC-WPRS Bulletin Vol.142, 2019 . 8-16 8
16.Wu, L.B., Holtkamp, F., Wairich, A., & Frei, M. 2019. Potassium Ion Channel Gene OsAKT1 Affects Iron Translocation in Rice Plants Exposed to Iron Toxicity. Frontiers in Plant Science, 10.
17.Bartak, M., Hajek, J., Morkusova, J., et al. 2018. Dehydration-induced changes in spec pectral reflectance indices and chlorophyll fluorescence of Antarctic e of Antarctic lichens with different thallus color, and intrathall intrathalline photobiont. Acta Physiologiae Plantarum, 40(10 10).
18.Bartak, M., Mishra, K.B., Mareckova A, M. 2018. Spectral reflectance indices sense desiccation induced changes in the thalli of Antarctic lichen Dermatocarpon polyphyllizum. Czech Polar Reports 8 (2): 249-259.
19.Gálvez, S., Mérida-García, R., Camino Ino, C. et al. 2018. Hotspots in the genomic architectu hitecture of field droughtresponses in wheat as breeding targets. Functional & Integrative Genomics.
20.Nuttall, J. G., Perry, E. M., Delahunt Ty, A. J. et al. 2018. Frost response in wheat and early detection using proximal sensors. Journal of Agrono f Agronomy and Crop Science, 205(2), 220–234.
21.Sytar O., Zivcak M., Olsovska K., Breststic M. 2018 Perspectives in High-Throughput Phenotyping of Qualitative Traits at the Whole-Plant Level. In: Sengar R., Singh A. (eds) Eco-friendly Agro-biolog logical Techniques for Enhancing Crop Productivity. Springer, Singapore.